- Bacteriology and Immunology
- Clinical Chemistry
- Medical Genetics
- Pathology
- Virology
- Transplantation Laboratory
- Research Programs
Contact Information
Haartmaninkatu 3 (P.O. Box 21)
FIN-00014 University of Helsinki
Finland
tel. +358 2941 911 (switch)

Further development of wetlab and in silico methods (Risto Renkonen Research Group)
We have already published the basic wetlab methodologies to be applied in this project and they include mRNA and microRNA extraction, labeling and gene chip hybridizations. HPLC purifications combined with automated nanospray MS and MS/MS will be run on our current ESI Q-TOF Ultima and the new high resolution MS to be purchased during 2009.
Mass spectrometry
Posttranslational modifications, such as glycan decorations, can dictate protein functions and thus have crucial importance in many processes such as inflammation. We have developed an automated in silico workflow to analyse mixtures of native glycopeptides with tandem mass spectrometry and an in silico workflow including database-related amino acid sequence identification followed by glycan analysis, and target-decoy filtering. Our workflow generates 105 putative interpretations from >109 theoretical glycopeptides. After scoring all these we got a validated interpretation with concomitant amino acid sequences, glycan compositions and structures for ten of glycoproteins in a single run. Instead of weeks and months of interpretation work of mass spectrometry files, our automated workflow can be run in a few hours and it provides information concomitantly from both amino acid and glycan moieties of intact glycopeptides in mixtures. This work has already begun with N-glycoprotein analysis, it will be expanded to O-glycoproteins and later to various other post translational modifications such as O-GlcNAc and phosphorylation analysis and will continue through out the project.
We continue to apply this novel technology to various analyses in regard to glycoproteins causing inflammation. Fukuda and Lowe recently showed that not only O- but also N-glycans dictate selectin-dependent leukocyte traffic into sites of inflammation in mice. As we study human patients, we will analyse the CD34 glycans in lymphatic tissues and at sites of inflammation. We analyse both the N- and O-glycans on both L-selectin binding and non-binding variants of human CD34 glycoproteins isolated from human tonsils. This work aims to pinpoint how the post translational modification regulates the inflammatory leukocyte extravasation into sites of inflammation in patients.
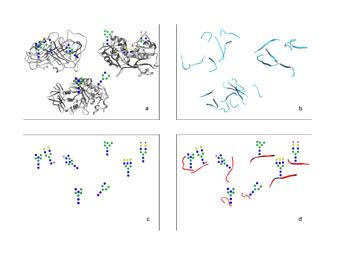
Fig 3. (a) Intact glycoprotein in a mixture to be analyzed. (b) Use of a pool of enzymatically digested peptides is the most common way to analyse proteomics, but no information of glycans is recorded. (c) Liberated glycans can be analyzed also, but then the links between the glycan and the peptide are lost. (d) Our novel computer-intensive automated workflow analysis of intact N-glycopeptides allows us to reconstruct the whole original N-glycoprotein structure even from a mixture of input glycoproteins.
