Hedman & Söderlund-Venermo Research group


Pathogenesis and Innovative Diagnosis of Infectious Diseases
We create innovative approaches for improved diagnosis of virus infections. We also assess the clinical impact and pathogenesis of the emerging human DNA viruses, parvo-, anello-, and polyomaviruses, by development of an extensive repertoire of molecular, immunological and diagnostic approaches for their in-vivo detection and their short- and long-term infection sequelae. We furthermore characterize the infectious agents themselves in molecular structure and function.
Early on we discovered in acute-phase rubella antibodies a peculiar characteristic, and developed for its measurement a new approach – the protein-denaturing IgG-avidity-EIA [Hedman, JID 1986, JCI 1988, JMV 1989, JID 1989]. Highly sensitive and specific for primary infection/immune response, our IgG-avidity methods have revolutionized the diagnosis of viral and protozoan infections – as shown for viruses of the hanta, parvo, paramyxo and herpes groups, as well as Toxoplasma gondii [Hedman, Lancet 1991; Söderlund, JID 1995a; Enders, JCV 2006, JID 2008; Hedman, JCV 2010; Meurman, JCM 1992; Paunio, Epidemiol Infect 2000; Korhonen, CDLI 1999; Lappalainen, JID 1993, PIDJ 1995, CID 1998; Roberts, EJCMID 2001; Buffolano EJCMID 2004; Hedman, JCV 2010]. Applied in several areas of basic research and epidemiology, the avidity assays have now also reached the commercial manufacturers, e.g., bioMérieux, Abbott and Roche.
With parvovirus B19 (B19V) -specific B-cell function our group has pursued the conformation dependence or epitope-type specificity of antibody-binding. With the major capsid protein VP2, we observed the IgG response for linear epitopes to be strictly confined to the acute & early convalescent phases – as opposed to the IgG response for conformational epitopes, prevailing for life. These findings gave rise to new diagnostic methods of three generations, further heightening the accuracy of B19V serodiagnostics [Söderlund, JID 1995b; Kaikkonen, JCM 1999, JMV 2001; Enders, JCV 2006; Enders, JID 2008], and disclosing the value of combinatorial serodiagnostics (IgM & IgG-quality) as a general principle.
Indeed, manufacture of innovative and reliable diagnostics based on in-house pro- & eukaryotic expression and molecular design of recombinant proteins is "daily life" in our lab, e.g. providing HUSLAB at our campus with high-quality diagnostics of many types. N.B., we have furthermore examined the virus-specific T-cell function against emerging human viruses such as the oncogenic Merkel cell virus (MCPyV), and the respiratory human bocavirus 1 [Franssila, JID 2001, Vaccine 2004; Kumar, Scand J Immunol 2011, PLoS ONE 2011, 2012].
In 1999 (first in Finland for any microbial pathogen) we set up a real-time quantitative PCR for sensitive and specific, early diagnosis and treatment-monitoring of the life-threatening post-transplant lymphoproliferative disease caused by EBV [Aalto, JCV 2003, CID 2007; Juvonen, BMT 2003, Haematologica 2007]. In regular clinical use since 2000, this method has saved many lives among the haematopoietic stem cell recipients at Helsinki University Central Hospital, just to mention a single site.
Our ex-vivo molecular studies have revealed the ubiquitous persistence of B19V-DNA genomes with intact wild-type sequence and full-length coding potential [Söderlund, Lancet 1997; Hokynar, JGV 2000]. We discovered a new B19V variant profoundly distinct in occurrence from all parvoviruses known – human B19 virus type 2 [Hokynar, Virology 2002; Ekman, JVI 2007]. We showed with large-scale molecular investigations that, the persistence of this virus in human tissues is life-long and represents a new "window into our past" – the Bioportfolio – showing that the "new" virus type 2 in fact is older in occurrence than the B19V prototype; i.e, both of the two circulated in Europe in equal frequency >50 years ago, whereafter virus type 2 disappeared from circulation [Norja, PNAS 2006].
Our group recently invented an efficient and robust method for generating bone marrow-derived erythroid progenitor cells (CD71+ CD36+) directly from unmanipulated human peripheral blood; a procedure of many apparent utilities in hematology and microbiology [Filippone, PLoS ONE 2010].
With human anelloviruses (prototype species TTV) residing in our tissues and cells as an exciting internal ssDNA microflora, we have constructed a full-length TTV clone and expressed all six TTV proteins for immunological studies and diagnosis. Our data suggested that the various TTVs in humans differ in antiviral antibody profiles, infection patterns and epidemiology. [Kakkola, JGV 2002 & Virology 2008; Chen, JGV 2013].
Much of our current research focuses on newly discovered human DNA viruses. With human bocaviruses (HBoV) 1-4, parvovirus 4 (PARV4), and four new human polyomaviruses including the carcinogenic MCPyV, we have set up comprehensive PCRs and recombinant-protein-based serodiagnostics (IgG; IgM; IgG-avidity), elucidating their epidemiology and clinical associations [Kainulainen, JCM 2008; Kantola, JCV 2009, JCM 2010, JID 2011; Söderlund-Venermo, EID 2009; Hedman, JCV 2010; Don, Ped Pulmon 2010; Chen, JCV 2011, JID 2011; Nascimento-Carvalho JMV 2012]. We showed that respiratory HBoV1 infections actually are systemic and viremic, can be diagnosed by PCR far better in serum than in nasopharynx, and do (contrary to many claims) cause symptomatic disease [Kantola, CID 2008; Söderlund-Venermo, EID 2009, Meriluoto, EID 2012]. We further showed the four human bocaviruses to cross-react serologically, and developed for them a new multiplex qPCR and a competition EIA for their specific antibodies [Kantola, JCM 1010, JID 2011]. PARV4 we showed to be extremely prevalent (78%) among North European i.v. drug users, and identified the first primary infections by this new blood-borne virus [Lahtinen, EID 2011].
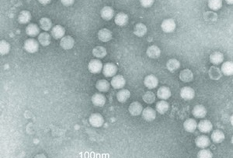
Our international collaborations have furthermore disclosed the comprehensive mRNA transcript map of TTV; and parvovirus B19 to have a remarkably high evolution rate of ~10-4 nt/site/year approaching that of RNA viruses; and pioneering evidence of circulation of boca- and PARV4-analogues in wild primates; and life-threatening respiratory disease or encephalitis due to human bocaviruses; and infectious HBoV1 clone-derived virions productively infecting polarized human airway epithelia (HAE), with hallmarks of lung injury [Qiu, JVI 2005, Norja, JVI 2008, Sharp, JVI 2010, Körner, EID 2011, Edner, JCM 2012, Mitui, CID 2012, Huang, PloS Pathog 2012].