| |
 |
Jokiranta Research Group
The complement system
The main functions of the complement (C) system are the elimination of microbes, the clearance of immune complexes, and the enhancement of the humoral immune response. C is activated through three initiation pathways: the classical, the lectin, and the alternative pathway.
 |
The alternative pathway
The alternative pathway (AP) is continuously activated at a low rate in human plasma. Plasma C3, the key component of AP, undergoes slow and spontaneous hydrolysis to C3(H2O). This provides a subunit for the initial C3 convertase of AP, C3(H2O)Bb, which cleaves fluid phase C3, generating metastable C3b. Within a short period this metastable C3b can attach covalently to any surface. The surface-bound C3b acts as a part of the C3 convertase, which cleaves and activates other C3 molecules. This leads to a positive feedback loop and progressively increasing deposition of C3b on the target surface. The AP amplification cascade causes effective C3b opsonization of foreign structures and simultaneous activation of the cytolytic terminal C cascade.
 Activation cascade of the alternative pathway of complement |
Recognition by the alternative pathway
Because the AP cascade does not recognize any target-specific activator structures, it must be strictly regulated. The main regulator of AP in plasma is factor H (fH). It acts as an accelerator of the decay of C3bBb and as a cofactor for factor I in the proteolytic inactivation of C3b. As AP is part of innate immunity, it must discriminate between activator and nonactivator structures to destroy only the harmful targets. This "recognition" is mediated by fH: when it has high affinity for surface-bound C3b the AP activation is stopped, but when it has low affinity the activation proceeds leading to efficient opsonization and lysis.
 Discrimination of the target by the alternative pathway |
Factor H
Factor H (fH) is an elongated molecule consisting of 20 short consensus repeat domains (SCRs) like a string of beads. The cofactor activity of fH has been mapped to the domains 14. FH has recently been reported to contain three binding sites for C3b, in SCR14, SCR815, and SCR1920 (Fig. 1). In addition, fH contains two binding sites for glycosaminoglycans (e.g. heparin or heparan sulfate) in domains SCR7 and SCR20, one for sialic acids in SCRs 1520, and two for C-reactive protein in SCR7 and SCRs 811. The existence of multiple interactions of fH with C3b and with the structures of self surfaces (sialic acids and glycosaminoglycans like heparin and heparan sulfate) serves as a basis for the target discrimination by the alternative pathway.
 Known binding sites of human complement factor H |
Atypical Hemolytic Uremic Syndrome and Carboxy-Terminal Part of Factor H
Atypical hemolytic uremic syndrome
(aHUS) is a familial disease characterized by erythrocyte fragmentation and
hematuria, damaged renal endothelium, vascular microthrombi, and
thrombocytopenia.
The syndrome leads ultimately to end-stage renal disease with high mortality
rate. In aHUS cases point
mutations have been found in complement components C3, factor B, CD46, factor
I, and factor H, all of which play a role in the activation or control of
the alternative pathway.
More than half of the mutations have been found to originate in the HF1
gene that encodes FH and FH-like protein 1, and the hotspot for the
mutations is within the region that endoces domains 19-20 of factor H.
We have solved crystal structure of FH19-20 (Jokiranta et al., EMBO J).
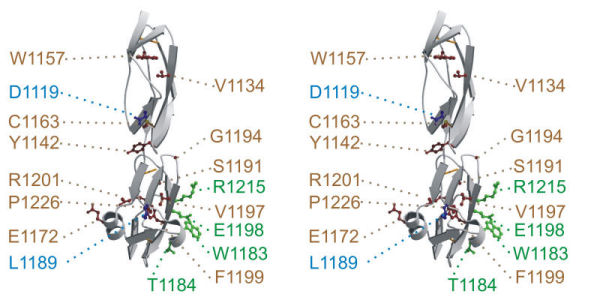 Stereo view of X-ray crystal structure of FH19-20 so that some aHUS-associated mutations are highlighted (for the color code see Jokiranta et al., EMBO J 1996). |
©2009 Jokiranta & Tiainen
Main Personnel Projects Publications Contact info Links Intranet pages