Advanced Microscopy unit
Department of Pathology
Haartman Institute, University of Helsinki
![]()
Facilities in AMU (continued)
Confocal microscopy
Leica-sp2 confocal microscope
Location: Room DK-139, Haartman Institute
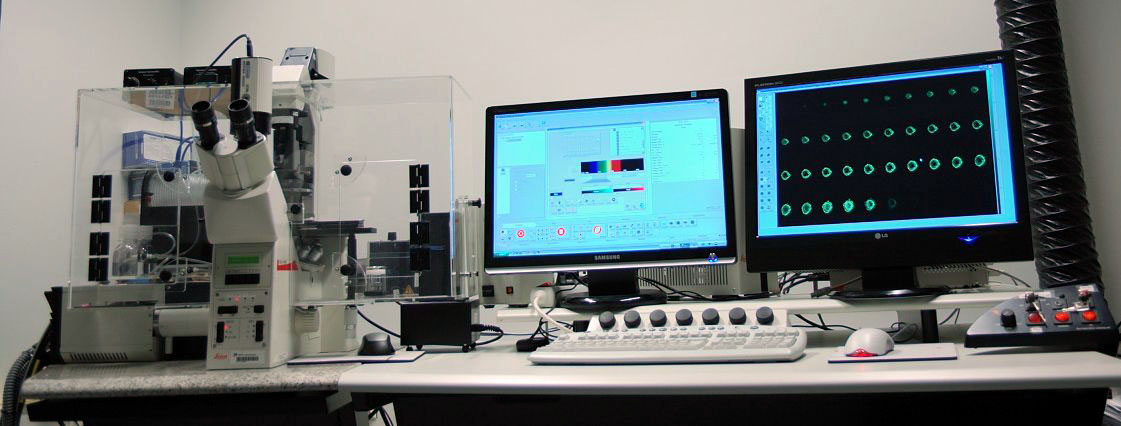
Laser line: Argon 458, 476, 488, 496, 514 nm five lines for blue
and green excitation, like CFP, YFP, GFP,
FITC etc., emission as green and yellow.
Krypton 568 nm for green excitation, like TRTIC, CY3, Alexa 567 etc, emission as
red.
HeNe 633 nm for red excitation, like Cy5, Alexa 647, emission as long red.
All laser lines wavelength selection
is controlled by AOBS, and laser intensity adjustment is controlled by AOTF:
totally filter-free control for excitation light sources.
Emission detection: An emission filter-free, spectral-meter based system for emission detecting. It detects emission within 400-750 nm in a continuously definable way, much versatile and less energy loss than conventional emission filter based system.
Microscope: Leica DM-IRE-2 inverted microscope motorized z-stage:
Fine-step z-stage stage: 160
µm total range / 40 nm per step.
Coarse-step z-stage: 2000
µm total range, 200 nm step.
Objectives: Dry lens: 2.5x/ 0.07;
5x/0.15; 10x/ 0.30; 20x/ 0.5.
Oil immersion lens: 40x/1.25; 60x/1.32; 100x/ 1.40.
All lenses are Fluoro, Plan corrected.
From 5x up, all lenses have Phase contrast, DIC (differential interference
contrast) optics equipped.
Environment chamber from SolentSci for living cell imaging including temperature control, temperature logging, CO2 enrichment control.
For taking advantage of the microscope's advance DIC, Phase contrast modes
and Fluorescent function, the microscope is also equipped with a high
sensitivity monochrome digital camera Retiga 2000 R. 2 mega pixels, pixel
size 7,4 µm.
With this camera, the non-laser illumination, wild-field microscopy can be
utilized to avoid laser cytotoxicity in long-time living cell imaging.
For conventional fluorescent microscopy: 3 fluorescence filter-sets are
installed.
Blue line: Ex. BP 450-490, Dichro.
510, Em. LP 515.
Green line: Ex. BP 546/12, Dichro. 565, Em.
BP 600/40
Red line: Ex. BP 620/60,
Dichro. 660, Em. BP 700/75
Fluorescent Microscopy
Zeiss Axiophot2 microscope with high sensitivity monochrome digital camera
Location: Room B224, B-wing, 2nd floor, Haartman Institute
 |
Objectives:
5x / 0.15 |
Filter set: DAPI |
| Digital Camera: Retiga 4000R (FireWire connection) 4 mega pixels, pixel size: 7.4 µ CCD chip size: 16.67 x 16.06 mm / 21.43 mm diagonal. The ccd chip size is the same as microscope's intermediate image plane, thus the truncating of image is avoided even without using photo-tube adaptor. Camera is also equipped with a RGB liquid crystal color filter slider for color imaging without compromising sensitivity of monochrome mode. |
||
Bright field Microscope for digital microphotography with high color fidelity
Location: Romm AK 126, Haartman Institute
This microscope features at very low magnification macro lenses down to 0.5x, and very good color fidelity in digital microphotography due to a special camera configuration.
 |
Objectives:
0.5x / 0.025 Macro
|
| Digital camera: Nikon D40 Digital SLR camera The pro-consumer camera is chosen on purpose because of its high color fidelity. In terms of color performances, It is much better than those scientific color camera I have ever used. For bright-field color imaging, the sensitivity is not a concern at all as light is much more than needed that you have to use ND filter to reduce it. When color fidelity is very important like for HE and other histochemical staining, this camera is recommended.
|
Video Enhanced DIC Microscopy:
Location: Room DK 139, Haartman Institute
Microscope and auxiliary parts:
Leica DM IRE-2 inverted microscope, shared with Leica SP2 confocal microscope with
all the DIC, Phase optical and fluorescence filter-set.
 Camera: Hammamatsu 2400-75i video CCD camera with on-chip integration function. On-line Digital image processor: Hammamatsu Argus-20, for on-line image acquisition, enhancement, processing tracing and analyzing. Connected to a PC by SCSI interface. Computer: A PC with enough capacity, running Windows 2000 for Argus-20 control software. Images captured from Argus-20 can be processed or edited by other program. |
Digital image analyzer:
Zeiss-KS400 digital image analyzer package
Microscope: Zeiss Axiokop upright
microscope
stage: x-y motorized stage.
Objectives: 5x, 10x, 20x, 40x, and
100x, chroma corrected.
Camera: Sony 960 3-CCD video camera, connected to Kontronik FG1 frame
grabber interfaced into a PC running Windows 3.11.
PC and image analysis software: The pc is, unfortunately, the old Windows
3.11 system and upgrading is impossible with the current frame grabber in use.
The software package still delivers all the necessary image enhancing,
processing, analyzing, statistical function, although with more inconvenience to
use.
SimplePCI image package:
Microscope, camera: share with KS-400 image analysis system.
Control PC: Same as VEC-DIC running under Windows 2000 but interface to the PC by a separate Matrox meteor II frame grabber.
Software package:
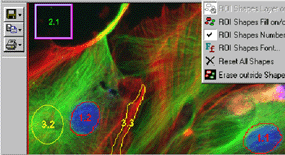 This package
is originally bought as the microscope control and image acquisition control
software for video enhanced DIC microscopy. But some function embedded in the
core module can be adopted for imaging analysis purpose. The core module
contains many binary image processing function and all the function for
classifying, counting and statistical operation. This package
is originally bought as the microscope control and image acquisition control
software for video enhanced DIC microscopy. But some function embedded in the
core module can be adopted for imaging analysis purpose. The core module
contains many binary image processing function and all the function for
classifying, counting and statistical operation. |
![]()
Statement about this web and
tutorial
For problems or questions regarding this web contact
webmaster
This page was last updated 08.12.2009